Targeted alpha therapy (TαT) is one of the most promising frontiers in cancer treatment. By using radioactive elements that emit highly energetic alpha particles, scientists can deliver lethal blows to cancer cells with extreme precision—damaging tumours while sparing surrounding healthy tissues. Among the most powerful tools in this approach is actinium-225 (Ac-225), a so-called “in vivo nanogenerator” that produces a cascade of radioactive daughter atoms inside the body. But there’s a catch: these daughters don’t always stay where they’re supposed to. A growing body of evidence suggests they may wander—and cause harm.
Two of these radioactive daughters, francium-221 (Fr-221) and bismuth-213 (Bi-213), are particularly important. While Bi-213 has already been used in clinical studies, Fr-221 has remained largely invisible. Its short half-life—just under five minutes—and chemical reactivity have made it extraordinarily difficult to isolate and study in living systems. As a result, our understanding of how these atoms distribute throughout the body—and potentially damage healthy organs—has been incomplete. Now, researchers have developed a new tool that makes the invisible visible.
In a collaborative study led by scientists from Bayer, the German Cancer Research Center (DKFZ), and the HUN-REN Centre for Energy Research, the team successfully created a new Ac-225/Fr-221 generator system. By using a specialised resin (LN2) and a gentle elution process at near-neutral pH, they were able to extract Fr-221 in a form suitable for injection into live mice. This seemingly technical advance proved transformative. For the first time, it became possible to trace the journey of free Fr-221—and its daughter Bi-213—throughout the body after injection.
The results were revealing. In tumour-bearing mice, Fr-221 showed a strong and rapid accumulation in the kidneys, salivary glands, and small and large intestines, with very little remaining in the bloodstream after just five minutes. In contrast, Bi-213 accumulated mainly in the kidneys and liver, showing a distinct but overlapping biodistribution. These findings suggest that radioactive daughters generated from Ac-225 don’t just disappear—they travel, settle, and may play a role in the side effects observed in patients undergoing alpha therapy.
The reason for this atomic escape lies in the physics of nuclear decay. When Ac-225 emits an alpha particle, the recoil kicks its daughter atom—Fr-221—out of any molecular cage or drug carrier designed to hold it. These free atoms then drift through the bloodstream until they find a new home, often in organs not originally targeted. This nuclear recoil effect is a challenge unique to alpha emitters and has long been suspected to contribute to off-target toxicity.
Understanding this redistribution is essential for designing safer and more effective radiotherapies. By mapping where these daughter isotopes go, scientists can better predict organ doses, refine their pharmacokinetic models, and potentially develop strategies to limit unwanted accumulation—such as improved chelators, protective agents for vulnerable tissues, or smarter drug delivery platforms that account for recoil dynamics.
This study offers a crucial piece of that puzzle. By shedding light on the biodistribution of previously untraceable radioactive daughters, the new generator technology enables a more complete picture of what happens in the body during alpha therapy. While more work is needed to translate these findings from mice to humans, this research marks a significant step toward precision-guided radiotherapy—where not just the bullets, but the shrapnel, can be tracked and controlled.
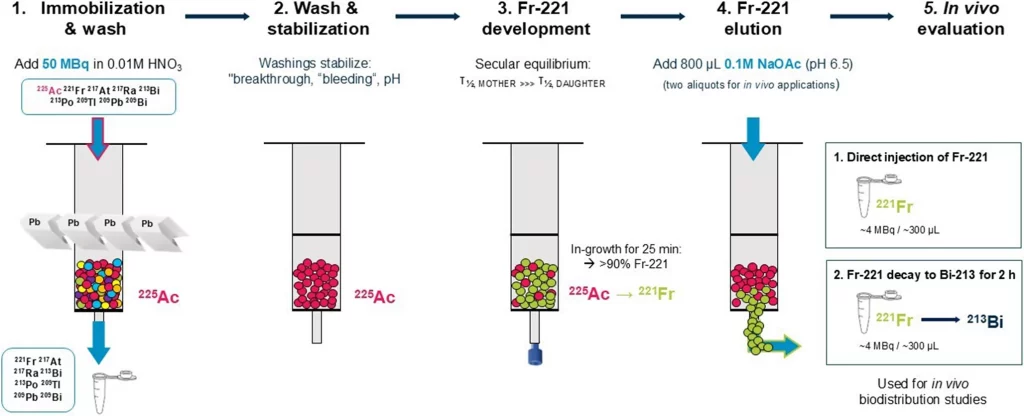
Figure reproduced from Zitzmann-Kolbe et al. (2025), Eur J Nucl Med Mol Imaging, under CC BY 4.0.
The study is published in the European Journal of Nuclear Medicine and Molecular Imaging and is available at https://doi.org/10.1007/s00259-025-07427-4.
Zitzmann-Kolbe, S., Remde, Y., Moen, I., Madas, B., Mázik, L., Suurs, F., Happel, S., Schäfer, M., Schatz, C., Taş, H., Hagemann, U.B., Benešová-Schäfer, M., 2025. Biodistribution of Free Francium-221 and Bismuth-213 in Tumour-bearing SCID Mice after Successful Development of Actinium-225/Francium-221 Radionuclide Generator Set-up. European Journal of Nuclear Medicine and Molecular Imaging. https://doi.org/10.1007/s00259-025-07427-4